The world's first fully automated synthesis module
The automated DESK-LAB process:
High quality
Ensures a high quality reproduction of each radiopharmaceutical batch
Safety
Reduces and simplifies the handling of radioactive material by the radiochemistry lab scientists
Automated GMP synthesis at your desk!
CISSPECT DESK-LAB represents a great leap forward for the development of radiopharmaceuticals and personalised drug discovery. Recently, we have developed the world’s first fully automated synthesis module, CISSPECT DESK-LAB, for the synthesis of Pt-195m cisplatin and other platinum compounds
The module has been designed for the production of GMP Pt-195m Cisplatin to facilitate a human pilot study. We have achieved this in close collaboration with our industrial partner FutureChemistry and renowned Dutch Academic Medical Centres.


Why automated synthesis?
The synthesis of a radiopharmaceutical can be a difficult process, with a series of reactions before getting to the final product. In the case of CISSPECT, there is a total of 7 steps required, until the desired molecules have formed in order to be administered to patients. At each step, it is of great importance that exact amounts are added to Platinum intermediate compounds, for a complete reaction. By using specific techniques developed together with our partner, the 7-step process has been automated in the DESK-LAB module, which ensures a high quality reproduction of each batch of the radiopharmaceutical under investigation. In addition, it reduces and simplifies the handling of radioactive materials by the radiochemistry lab scientists and within a Hot Cell with limited access.
Results
Fully automated synthesis of Pt-195m cisplatin
For the GMP production of various Platinum compounds
Automated dispensing into patient doses
In addition to the Dhara synthesis according to Hoeschele et. al., Radiochimica Acta 1982

Partner: FutureChemistry
FutureChemistry Holding B.V. develops, builds and implements equipment and processes for manufacturing of chemicals with a high added value. With a focus in particular on two product groups: nanomaterials and radiopharmaceuticals.
- Located at the Technology and Science Park of the Radboud University Nijmegen
- Years of experience in flow chemistry
A great leap forward for the development of radiopharmaceuticals and personalised drug discovery.
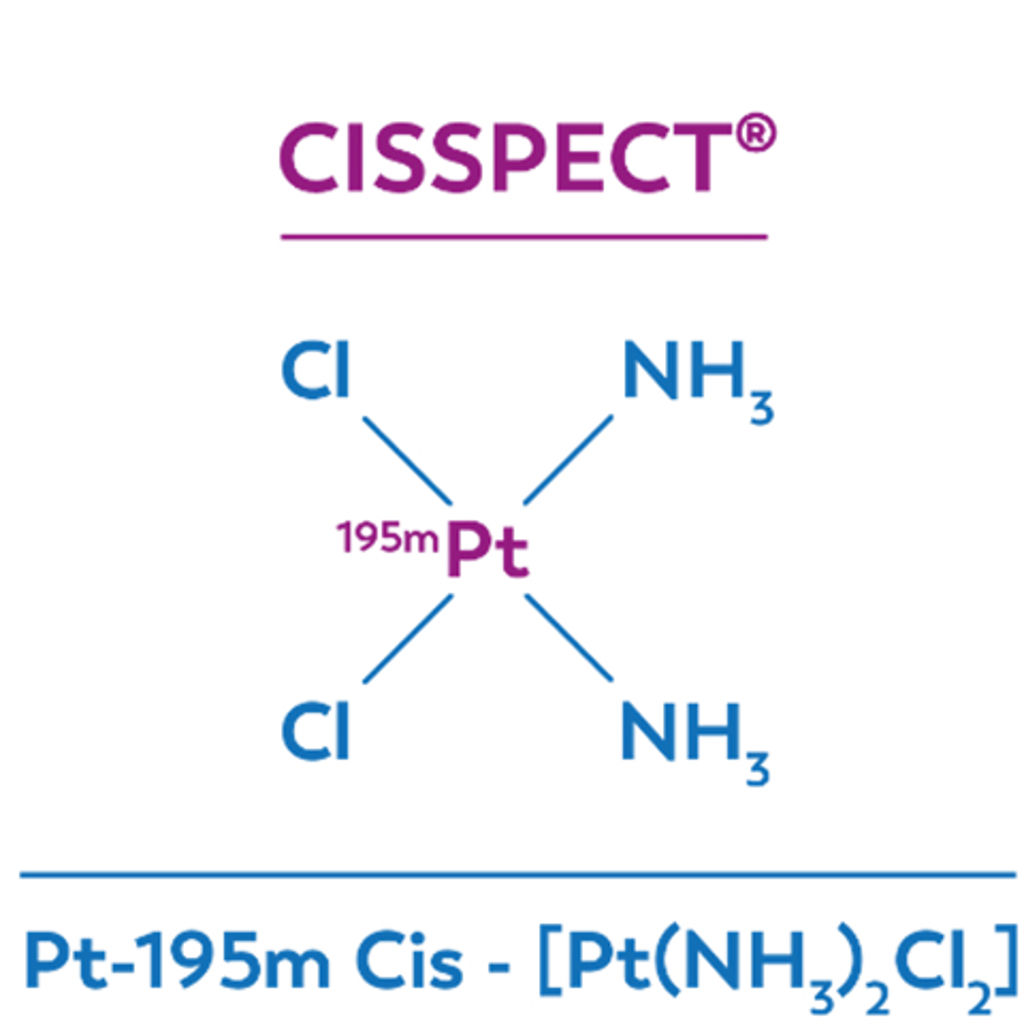
CISSPECT = Pt-195m = radioactive cisplatine
The main draw backs of cisplatin chemotherapy treatment is kidney toxicity, as well as the low response rate in many cases, which cannot be predicted beforehand. Pt-195m cisplatin, or CISSPECT, is developed to enable quantification of the in vivo bio distribution of cisplatin, a standard chemotherapeutic agent. Pt-195m cisplatin may be able to select patients who will not respond to cisplatin chemotherapy, or those patients who will suffer from severe kidney toxicity due to cisplatin treatment.
CISSPECT® DESK-LAB - Automated GMP synthesis at your desk!
Human Pilot
The feasibility of SPECT imaging and quantification of Pt-195m in a preclinical setting has already been shown (E.A. Aalbersberg et al., EJNMMI 2017 (44), 8, 1347 – 1354). As a next step, the feasibility in humans will be assessed. If successful, Pt-195m cisplatin contributes to optimising treatment outcome of chemo(radio)therapy and reducing toxicity in a personalised approach.
Relevant products
More information?
We're happy to help you.
Marjolijn Droog










